
Interest in the potential use of algae butanol as a feedstock in biofuel production is gaining momentum in the United States and Europe. The reasons for the interest in algae is because of their ability to grow on marginal land, the high concentrations of carbohydrates and lipids in their cell mass , and the ability to clean nitrogen and phosphorus contaminants from water.
In fact, ongoing research at the University of Arkansas has found that the nitrogen and phosphorous in the Mississippi River could provide a source for as much as 250 million gallons of biofuel per year, while also providing a cleaner water source. However, although biodiesel from algae has been studied extensively, there have been far fewer studies on the conversion of algae to fuel oxygenates and no studies on the conversion of algae to butanol. Since the DOE has identified biobutanol as a 2nd generation biofuel, research of converting algae into butanol is important and could lead to a sustainable fuel alternative.

Specific research aim was to transform native algae strains, grown to clean contaminated water, into butanol (1-butyl alcohol). The algae used were from sources inside of Arkansas and New York City.
The first process step was drying the algae. It was found that enough water could be removed in 2-3 days by air drying in a greenhouse to make algae dry enough for subsequent processing.
The next processing step was to hydrolyze the algae and extract carbohydrates for ultimate butanol production. We found that at a temperature of 110 C, a short time (30 minutes or less) combined with a high acid concentration (as high as 30 g/L) was optimal for maximum carbohydrate production.
The next processing step was to ferment carbohydrates into butanol using clostridium spp. It was found, however, that C. sacchroperbutylacetonicum was suitable for growth and was able to produce butanol from algae. We also found that the butanol could be separated efficiently using a 2-step distillation with phase separation.
Algae butanol is a viable alternative to ethanol as a fuel oxygenate for gasoline, and has advantageous benefits as a liquid fuel in three key areas:
- Low vapor pressure (loss due to evaporation is decreased).
- High energy density (less volume required for storage).
- Compatible with existing infrastructure (issues of blending and transport eliminated).
However, butanol production by the fermentation of sugars is a complex process and requires significant development to make it commercially feasible. Three major problems limit the commercial application of the carbohydrate to butanol process:
- Typical fermentation products from a carbohydrate to butanol fermentation include a mixture of organic acids and alcohols, as well as hydrogen and CO2
- The fermentation also suffers from product inhibition, which limits the cell concentration, yield and concentration of butanol in the product stream.
- Butanol is partially miscible in water, forming two liquid phases in equilibrium with a single vapor phase.
Potential problems with the use of algae butanol fuel

The potential problems with the use of algae butanol are similar to those of ethanol:
- To match the combustion characteristics of gasoline, the utilization of butanol fuel as a substitute for gasoline requires fuel-flow increases (though butanol has only slightly less energy than gasoline, so the fuel-flow increase required is only minimal, maybe 10%, compared to 40% for ethanol.)
- Alcohol-based fuels are not compatible with some fuel system components.
- Alcohol fuels may cause erroneous gas gauge readings in vehicles with capacitance fuel level gauging.
- While ethanol and methanol have lower energy densities than butanol, their higher octane number allows for greater compression ratio and efficiency. Higher combustion engine efficiency allows for lesser greenhouse gas emissions per unit motive energy extracted.
- Butanol is one of many side products produced from current fermentation technologies; as a consequnece, current fermentation technologies allow for very low yields of pure extracted butanol. When compared to ethanol, butanol is more fuel efficient as a fuel alternative, but ethanol can be produced at a much lower cost and with much greater yields.
- Butanol is toxic at a rate of 20g per liter and may need to undergo Tier 1 and Tier 2 health effects testing before being permitted as a primary fuel by the EPA.
Current use of butanol in vehicles
Currently no production vehicle is known to be approved by the manufacturer for use with 100% butanol. As of early 2009, only few vehicles are approved for even using E85 fuel (i.e. 85% ethanol + 15% gasoline) in the USA.
However, in Brazil all vehicle manufacturers (Fiat, Ford, VW, GM, Toyota, Honda, Peugeot, Citroen and others) produce flex fuel vehicles that can run on 100% ethanol or any mix of ethanol and gasoline. These flex fuel cars represent 90% of the sales of personal vehicles in Brazil, in 2009. BP and Dupont, engaged in a joint venture to produce and promote algae butanol fuel, claim that “biobutanol can be blended up to 10%v/v in European gasoline and 11.5%v/v in US gasoline.
You might also be interested in...
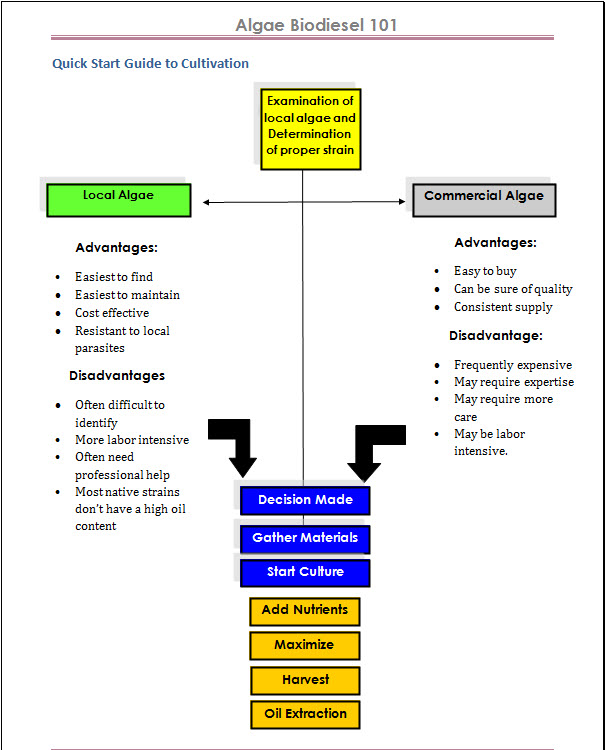
The Algae Biodiesel Process
The algae biodiesel process is fairly straight-forward, however the devil is in the details. Read More

The Two Biggest Mistakes in Algae Biofuels
Without a doubt, The Two Biggest Mistakes I saw as a biofuel consultant in advanced biofuels were...Read More
Algae Biofuels: Separating Myth From Fact
Lot so wild stories and wild claims being made in the algae biofuels space. Sifting through the flotsam of cyberspace isn't easy... Read More
The Algae Revolution Has Begun
David, just read through your book on algal biodiesel. Nice layout, easy to read with emphasis on building from local materials and good optimism. We may use some of these ideas for our engineering senior design course this year on biofuels production. Thanks..
Professor of Biosystems Engineering



